Resonance Structure No3
Consider the reaction and its rate law. Molecular orbital theory- Methodology Orbital energy level chart Bond request Magnetic properties for homonuclear diatomic species.

Resonance Structures For No3 Nitrate Ion Youtube
Zhiyi Wei Binbin Zhou Yongdong Zhang Yuwan Zou Xin.
. Boosting Hydrogen Evolution Electrocatalysis via Regulating the Electronic Structure in a CrystallineAmorphous CoPCeO x pn Heterojunction. Pulsed Multifrequency Electron Paramagnetic Resonance Spectroscopy Reveals Key Branch Points for One- vs Two-Electron Reactivity in MnFe Proteins. Thomson and Rutherford atomic models and their limitations.
The nitrate ion carries a formal charge of 1. Solution for bond hybridization on formal charge for on central atom element in Bold Lewis structure Molecular Shape angles 1. Valence Bond Theory- Orbital overlap Directionality of bonds.
When writing the chemical formula for an ion its net charge is written in superscript immediately after the chemical structure for the moleculeatom. For analytical study of the prepared sample the amount of absorption within wave length of 300550 nm was observed by uv-vis spectroscopy. Cations Pb2 Cu2 Al3 Fe3 Zn2 Ni2 Ca2 Ba2 Mg2 NH4 Anions- CO3 2 S 2- SO4 2 NO3- NO2- Cl- Br- I- Insoluble salts excluded.
Although single atom catalysts SACs have gained interest for the electrochemical reduction reactions of both carbon dioxide CO 2 RR and nitrate NO 3 RR the structureactivity relationship for Cu SAC coordination for these. We have found that different conductive strategies of MOFs fundamentally depends on chemical structure and interaction between the metal ions and organic ligands 4 10 424344 54 59 60Commonly doping strategies for MOFs provided an excellent structure stability based on its chemical composition 111213 15 59 61Therefore we have majorly. Shafaat Journal of the American Chemical Society 2022 144 27 11991-12006 Article Publication Date Web.
This charge results from a combination formal charge in which each of the three oxygens carries a 2 3 charge whereas the nitrogen carries a 1 charge all these. The spectrum of the hydrogen atom Bohr model of hydrogen atom its postulates derivation of the relations for the energy of the electron and radii of the different. Sodium nitrate features an ionic bond between one Na ion and one NO 3 ion.
Enthalpy of solution of CuSO4 2. The net charge is written with the magnitude before the sign. Discovery of subatomic particles electron proton and neutron.
The negative charge on this ion is delocalized due to resonance. The structure of a NaNO 3 molecule is illustrated below. It is known that an absorption band at about 370 nm due to surface plasmon resonance in ZnO nanoparticles.
Unit 3 Atomic Structure. The nitrate anion has a trigonal planar structure in which 3 oxygen atoms are bonded to a central nitrogen atom. Nature of electromagnetic radiation Photoelectric effect.
Carbon tetraflouride 2 N. Determine the concentration. Lewis picture reverberation structures VSEPR model molecular shapes Covalent Bond.
A precipitate of PbI is formed when 5 mL of 0012 M PbNO is added to 5 mL of 0030 M KI. Kisgeropoulos Yunqiao J. Xiao-Feng Wang ACS Applied Materials Interfaces 2022 14 29 33151-33160 Energy.
Click to see the answer. Chemical principles involved in the following experiments. Xue-Zhi Song Wen-Yu Zhu Jing-Chang Ni Yu-Hang Zhao Tao Zhang Zhenquan Tan Li-Zhao Liu and.
A solution with a total volume of 750. 1 shows the UV-Vis spectra of ZnO nanoparticles recorded between 300 and 550 nm. Closing both the carbon and nitrogen loops is a critical venture to support the establishment of the circular net-zero carbon economy.
That is a doubly charged cation is indicated as 2 instead of 2However the magnitude of the charge is omitted for singly charged moleculesatoms. 4 A 3 B products kAB. Click to see the answer.
The ion is the conjugate base of nitric acid consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. Narrow your search by program title school name location and more. Enthalpy of neutralization of strong acid and strong base.
Chemical principles involved in the qualitative salt analysts. ML contains 371 g MgNO32.
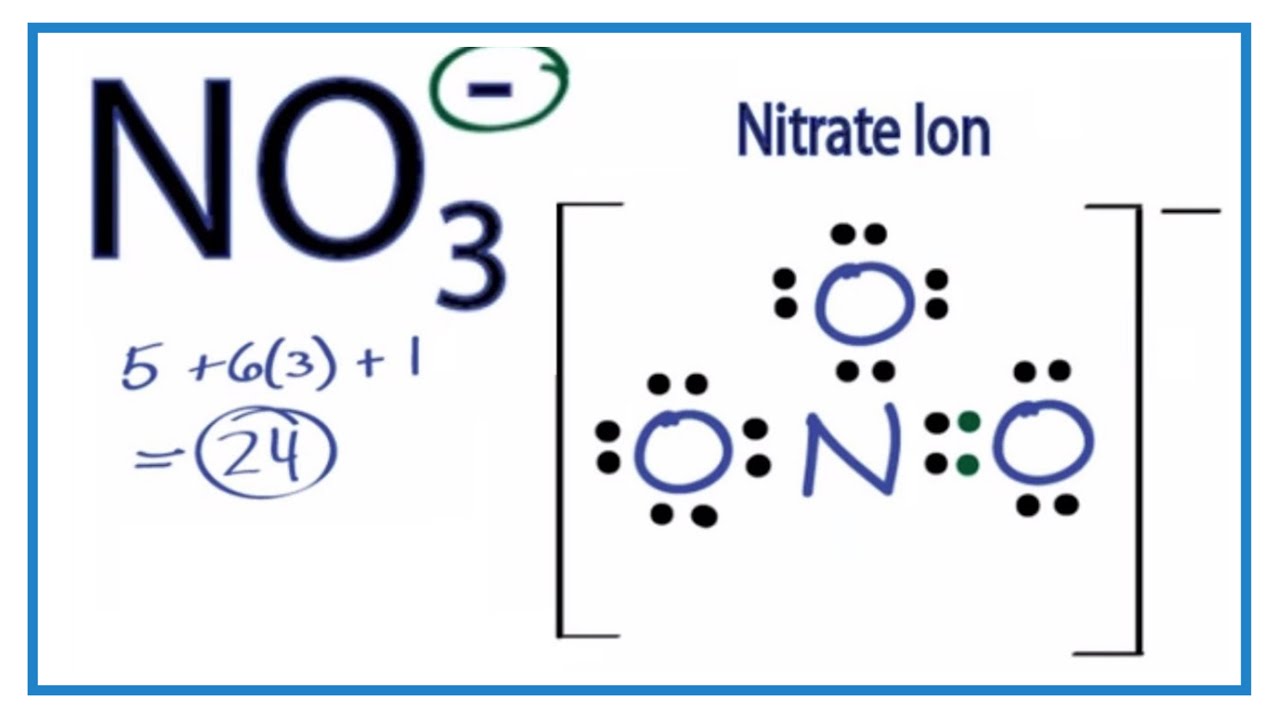
No3 Lewis Structure How To Draw The Lewis Structure For No3 Youtube
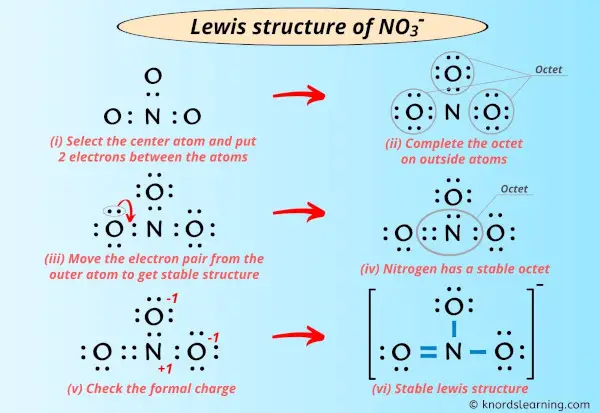
Lewis Structure Of No3 With 6 Simple Steps To Draw
Resonance Structures And Calculated Mulliken Charges Of No 3 A Download Scientific Diagram

No3 Lewis Structure Molecular Geometry Details Overview Tuktuk Study
Comments
Post a Comment